Umay™ Care
An eye therapy device for digital eye strain.Treating Digital Eye Strain

Umay™ Care contracted Tangent to come up with a design for a rechargeable device that could both heat and cool the eyes. Tangent’s experience was indispensable for meeting full regulatory compliance as a class II medical device. The device had to be safe, comfortable to wear, and fool-proof.
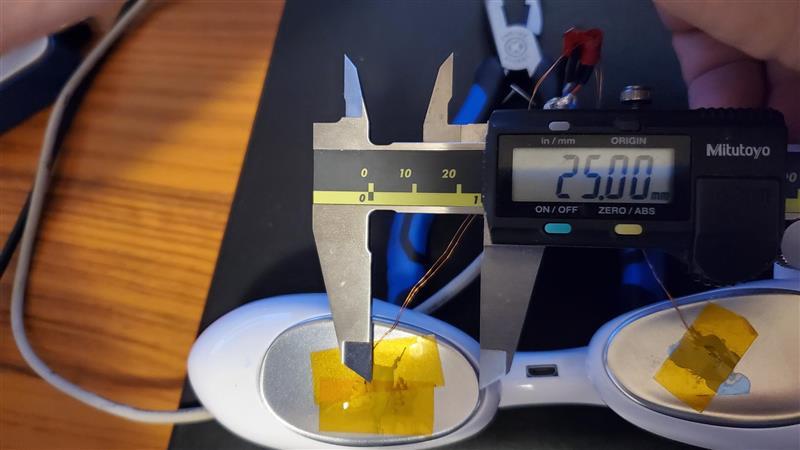
Tangent took on the development of an alpha prototype, involving work across our teams of mechanical, electrical, firmware, and industrial design. We then put the prototype up against rigorous durability tests while thermal simulation software studied the temperature of a virtual eyeball, all making sure the device was performing as it was meant to. For months, a mannequin head used for testing wore the Umay Rest prototype and made its way around the Tangent office
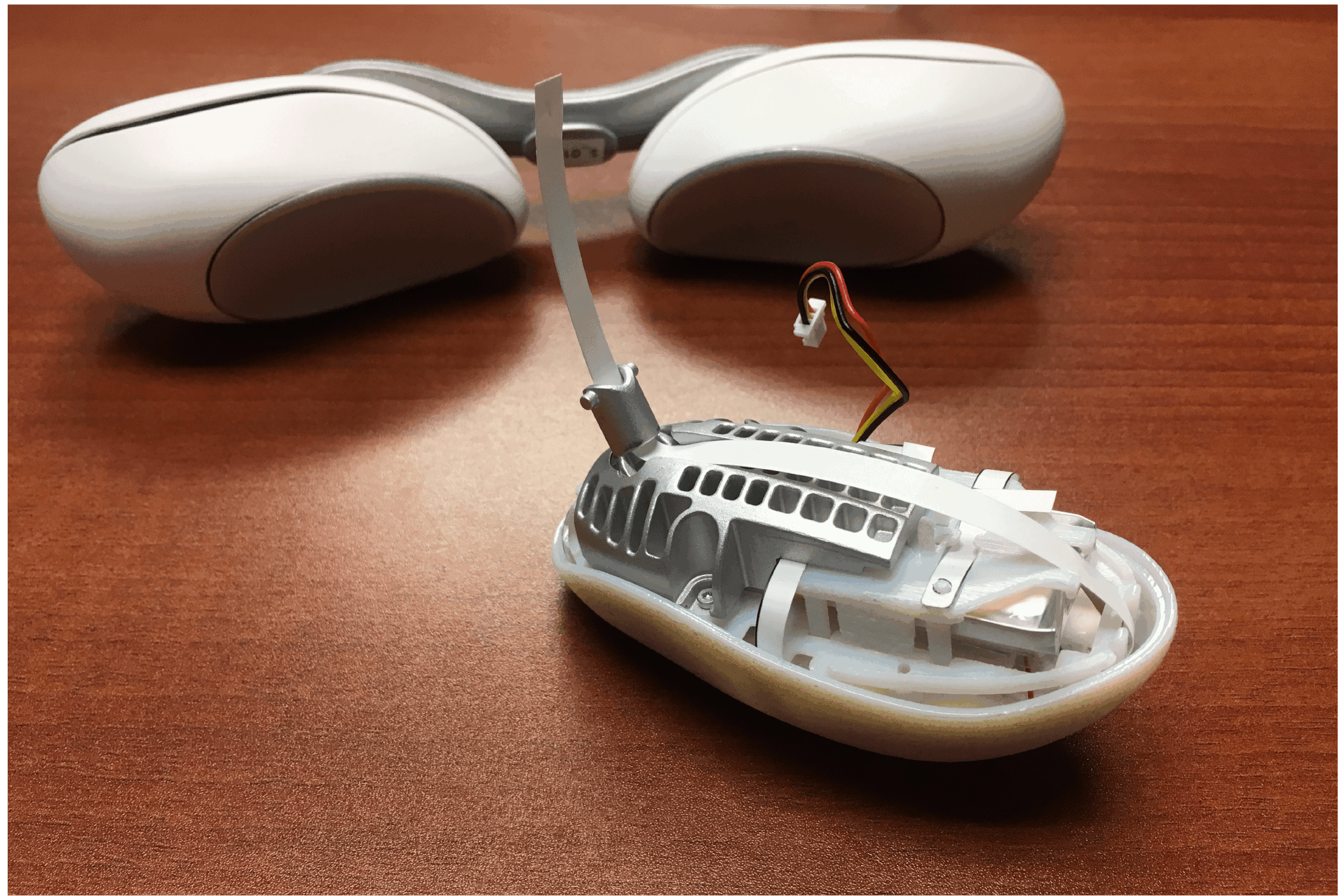
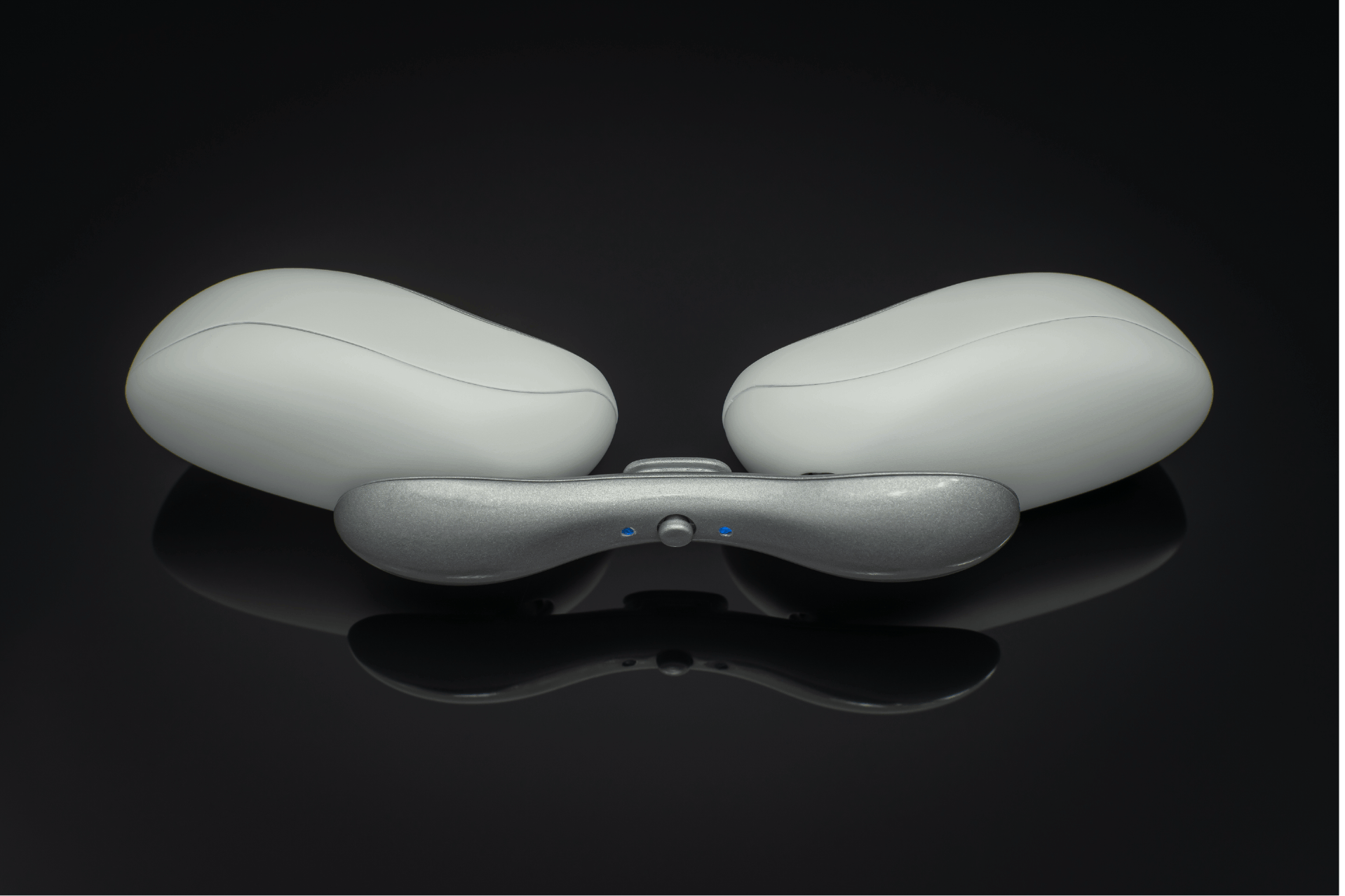
We ended up with a sleek, compact device as a beta prototype, which became the basis for an initial low-volume production run for in-field testing. Tangent was able to design a fully adjustable, wearable device that met the client’s required specifications, including using a medical-grade charger (USB-C), hitting the target weight of 160 grams, and having a beautiful, organic form.
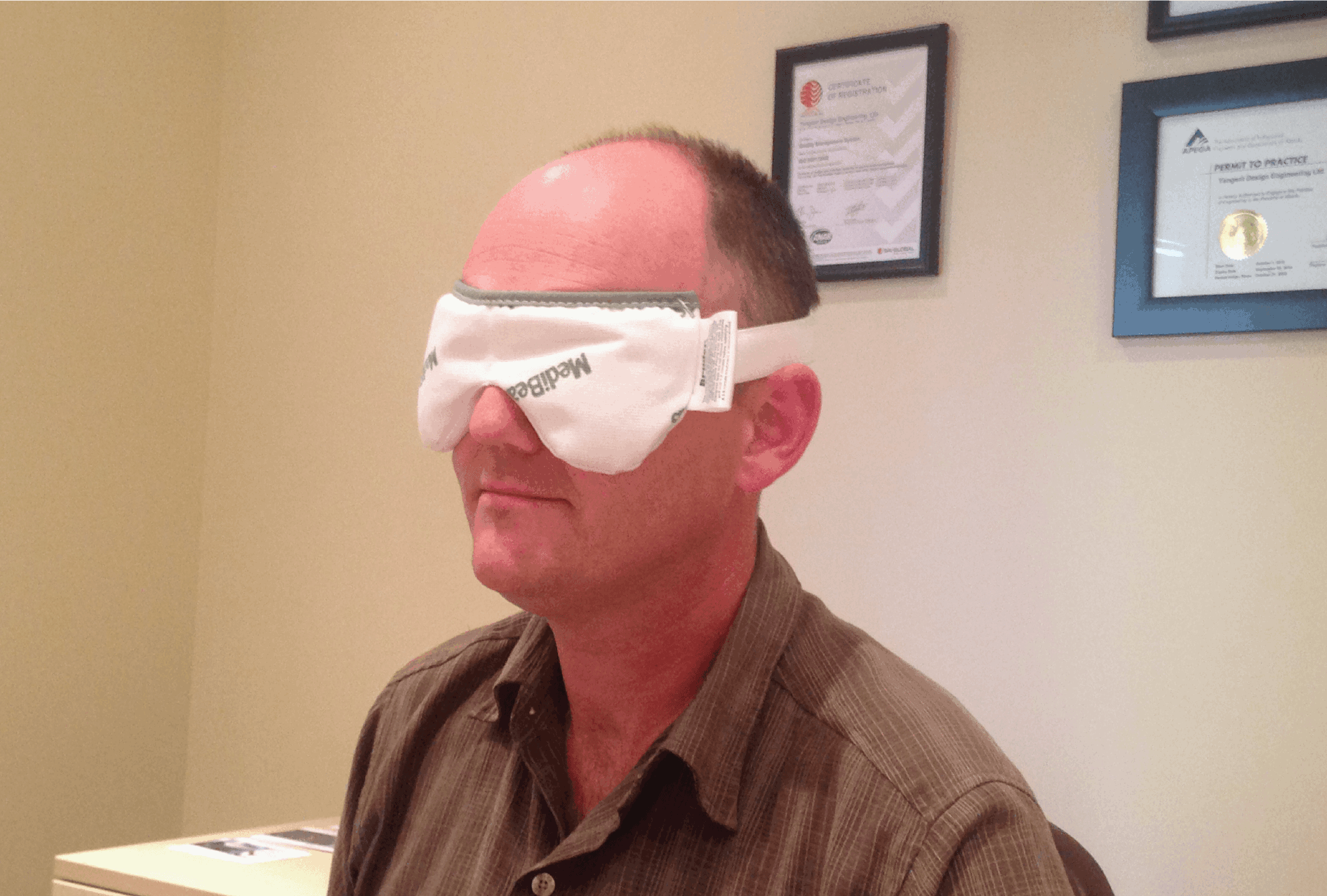
We have now supported Umay™ Care through a phase of design for manufacture. With our years of experience, we continue to act as a key technical resource for finding a contract manufacturer while upholding the integrity of Umay Rest’s design.